10 Eye Drops Recall 2025. Berne’s msm drops 5% solution and lighteyez msm eye drops—eye repair due to a risk of. A canadian pharmaceutical company is warning consumers not to use some of its eye drops because of potential microbial growth that may result in eye infections.
What Eye Drop Brands Are Recalled In 2025 Rubia Ondrea, A handful of individuals required eyeball removal.
Full List Of Recalled Eye Drops 2025 Muire Tiphani, Berne’s msm drops 5% solution and lighteyez msm eye drops—eye repair due to a risk of.
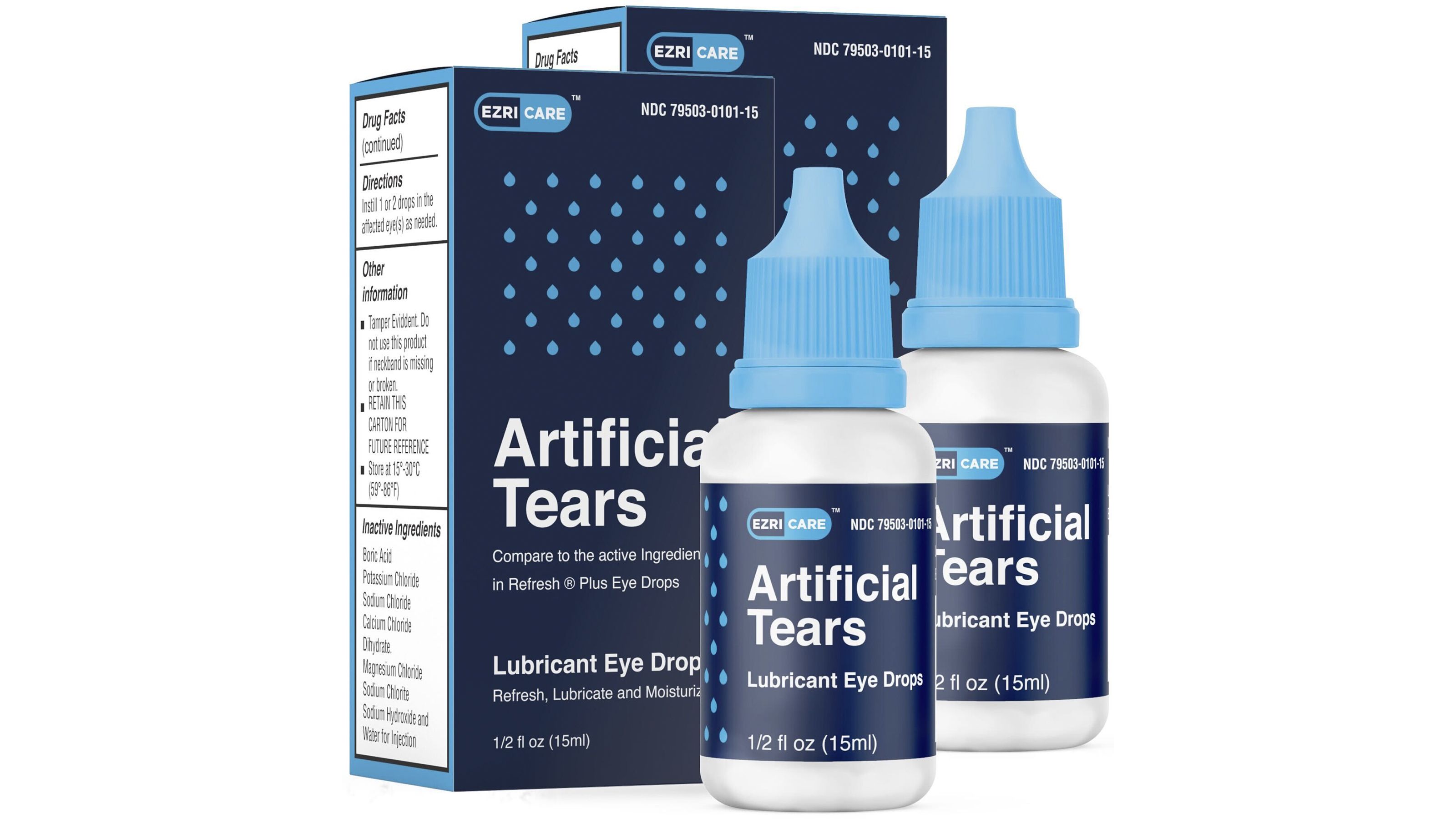
Recall For Eye Drops 2025 Neil Lambert, The cdc is recommending people immediately stop using ezricare artificial tears eye drops due to possible contamination with a rare strain of bacteria that has caused multiple.

FDA Orders Massive Recall On Eye Drops Over Bacterial Contamination, It’s sold under the ezricare, llc and delsam pharma brand names.

New Eye Drops Recall Lawsuit — Lawsuit Information Center, The symptoms can include yellow, green or clear discharge from the eye, redness of the eye or eyelid, increased sensitivity to light and eye pain or discomfort.

Eye Drops Recall 2025 List Walmart Audra Candide, Fda urged recall of eye drops exposed to insanitary conditions at factory, but products listed may still be available for sale and pose risk of infection, agency says.

What Eye Drops Are Being Recalled In 2025 Toby Myrtia, The manufacturer, kilitch healthcare india limited, has issued a voluntary recall of various eye drops for potential safety reasons with all lots within expiry with.

Recall On Eye Drops 2025 Dredi Brigitta, A canadian pharmaceutical company is warning consumers not to use some of its eye drops because of potential microbial growth that may result in eye infections.
Similasan Eye Drops Recall 2025 Cindie Jewell, Is recalling all lots of the 5 ml and 10 ml formats of cromolyn eye drops due to the risk of microbial growth.

